|
|

Structure,
Sorption, and Reaction Dynamics in
Petrochemical Catalysts:
Theoretical & Experimental Studies
Jumras Limtrakul, Supa Hannongbua,
Chak
Sangma, Pensri Boonsawansong, Piboon Pantu
Chan Intam, Chadchalerm Raksakul, Jakkapan
Sirijaraensre, Kanokthip Srisuk, Piti
Treesukol Sombat Ketrat, Somkiat Nokbin,
Virasak Dungsrikaew, Tanin Nanok, and
Zahree Rakpattani
Laboratory
for Computational and Applied Chemistry,
Physical Chemistry Division, Chemistry
Department,
Kasetsart University, Bangkok, Thailand
http://lcac.ku.ac.th
| Introduction |
| |
Heterogeneous catalysis
is an utmost important area
of research that has direct
impact on the chemical industries.
Currently, the environmental
impacts of industrial processes
are becoming important issues,
meaning that moving toward replacing
liquid acid/base catalysts with
novel solid catalysts is receiving
great attention. Unlike liquid
acid/base catalysts, solid acid/base
catalysts do not produce significant
amounts of waste byproducts,
making them more environmentally
sound. One of the most recognized
heterogeneous catalysts are
zeolite-type catalysts which
are ideal catalytic materials
for many processes because of
their well-defined nanostructure
with pore sizes in the molecular
scale, thus enabling unprecedented
shape selected chemistries.
Zeolites are now widely used
in petroleum refineries and
petrochemical industries and
fine chemical productions. It
has been estimated that the
employment of zeolites in petroleum
refining alone resulted in an
added value of several billion
US dollars per year. Although
experimental techniques provide
us many informative data, using
these techniques to study heterogeneous
catalysts and their reactions
is quite limited. With the advance
in computer technology, scientific
equipment and theoretical methodology,
it has become apparent that
molecular modeling provides
a cost effective route to enhance
the competitiveness of these
industries. Understanding reactivity
of zeolites has been a scientific
challenge from both experimental
and theoretical points of view.
Our focus is to study mechanisms,
kinetics and dynamics of reactions
in zeolites using experimental
and theoretical methodologies
in parallel. The development
of innovative crystalline materials
is of our interest as well.
Chemical Physics Letters 2001 350
(1,2) 128-134; J. Phys.
Chem. B. 2001 105 (12)
2421-2428; J. Catal.
2000 153 (1-2) 155-163;
Chem. Phys. 1997
215 (1) 77-87.
|
|
| The topics on which we are currently focusing
are: |
| The
protonation reaction of unsaturated
hydrocarbons within zeolites:
|
| |
Of the most interest
in this active research is the
protonation reaction of ethylene
with Brønsted zeolites
that is the foundation step
of several industrially important
reactions, namely the polymerization
and hydrocarbon cracking processes.
It was found that the electrostatic
contribution is a major part
of long-range interactions.
This raises the need for a better
understanding on the effects
of the Madelung potential in
the potential energies of reaction
intermediates.In this study,
the embedded cluster approach
is used to study complete protonation
reaction of ethylene within
both H-FAU and H-ZSM-5 zeolites.
Cluster model calculations of
the reactants and intermediate |
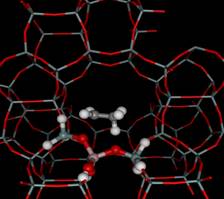
Transition state of ethylene
protonation
on H-ZSM-5
|
| |
complexes
have been compared with results
obtained at the same level of
theory using embedded cluster
approach. The apparent activation
energy is evaluated to be 14.6
and 17.9 kcal/mol for the ZSM-5
and FAU, respectively and can
be compared well with the experimental
apparent activation energy of
16 kcal/mol determined for deuterium
exchange of ethylene on a Y
zeolite. Chemical Physics Letters, (2001)
349 (1,2) 161-166; Stud.
Surf. Sci. Catal., (2001)
135, 2469-2476. |
|
| Synthesis,
Characterization and Modelling
of Ga-MFI-type Zeolites:
|
|
|

Scanning
electron micrographs of
Na/Ga-MFI-type zeolite: Si/Ga=100 |
A combined study employing
both theoretical and experimental
methods has been carried out
for the first time to investigate
the structures and bonding of
gallosilicate MFI-type (ZSM-5)
zeolites. The hydrogel compositions
with varying Si/Ga ratios of
25, 50, 75 and 100 were 62Na2O
: xGa2O3 : 60SiO2 : 4TPABr :
3134H2O : 60NaCl (x=1.2, 0.6,
0.4 and 0.3). The four Ga-MFI-type
zeolites were synthesized by
a rapid crystallization method
in 1 hour. The obtained solids
were characterized by XRPD,
SEM and FT-IR. The results reveal
that the profile corresponded
to of MFI structure and that
no crystalline impurity was
present in the products. The
Brønsted acid strength
of various cluster models representing
H/Ga-MFI-type zeolite has been
carried out at density functional
theory (DFT) and |
|
| |
Hartree-Fock
(HF) levels of theory. We have
investigated the influence of
its composition on the structure
and acid strength by varying
Si/Ga ratios of 13 (cluster
A), 15 (cluster B) and 17 (cluster
C). Changes in Si/Ga ratio have
a great effect on the proton
affinity of hydroxyl proton
due to the long-range electrostatic
interaction. Our calculated
results estimate that the acid
strength increases in the order:
cluster A < cluster B <
cluster C, which exhibit an
exceptional agreement with both
previously experimental and
theoretical studies.
2002 Patented.; J.
Mol. Struct.1999 510
(1-3) 131-147. |
|
| Ethylene Epoxidation
over Ti-substituted Silicalite
(TS-1): |
| |
Mechanism of the ethylene
epoxidation with hydrogen peroxide
over Ti-substituted silicalite
(TS-1) catalyst was investigated
by using both the quantum cluster
and embedded quantum cluster
approaches. The predicted structure
of TS-1 is in a good agreement
with the experimental results.
The epoxidation of ethylene
consists of two steps.
First, the chemisorption of
H2O2 at the Ti active site forms
the oxygen donating Ti-OOH species
and then the transfer of an
oxygen atom from the Ti-OOH
species to the adsorbed ethylene.
The later step was found to
be the rate limiting step with
the barrier of 17.0 kcal/mol,
which is in good agreement
with the experimental estimate
|
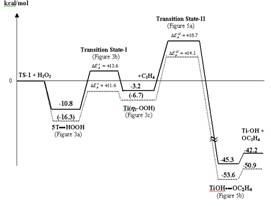
Schematic
energy profile for
the epoxidation of ethylene
by TS-1. |
| |
of
about 16.7 kcal/mol. This
result also shows that inclusion
of the effects of the zeolite
crystal framework is crucial
for obtaining quantitative energetic
information. For instance,
the Madelung potential increases
the barrier of the oxygen atom
transfer step by 4.6 kcal/mol.
Journal of Physical Chemsitry B 2002,
in press. |
|
| Beckmann
rearrangement: |
|
| |
Beckmann rearrangement
is an industrially important
reaction for producing e-caprolactam,
which is a raw material for
the production of Nylon-6 with
the market of 3.6 million tons
in 1998. Caprolactam is produced
by Beckmann rearrangement of
cyclohexanone oxime with oleum
or concentrated sulfuric acid
as a reaction medium. Although
this procedure is convenient
from the chemical standpoint,
corrosion difficulty of the
manufacturing equipment and
elimination a large amount of
ammonium sulfate formed during
the neutralization process make
the process environmentally
unacceptable. |
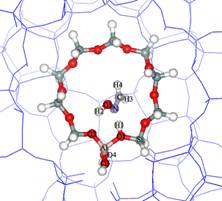
1,2
H-shift transition state |
| |
Then
using heterogeneous catalyst in
this reaction, usually called
the vapor phase Beckmann rearrangement,
can solve these problems. Zeolite
proves to be an excellent candidate
for taking over the catalytic
function, since the use of a zeolitic
catalyst is benefit not only from
an economical point of view but
also from an ecological viewpoint.
Journal of Physical Chemsitry
B 2002, in press. |
|
|
|



